Details of the Drug
General Information of Drug (ID: DM2JYQA)
| Drug Name |
S109
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
oxapium iodide; 6577-41-9; Cyclonium; Cyclonium iodide; UNII-682380CG4N; SH 100; 682380CG4N; DSSTox_CID_26925; DSSTox_RID_82021; DSSTox_GSID_46925; Esperan; Oxapii iodidum; Ciclonium iodide; Iodure d'oxapium; Ioduro de oxapio; 1-[(2-cyclohexyl-2-phenyl-1,3-dioxolan-4-yl)methyl]-1-methylpiperidin-1-ium;iodide; CAS-6577-41-9; NCGC00181356-01; Oxapium iodide [INN:JAN]; Oxapii iodidum [INN-Latin]; C22H34INO2; Iodure d'oxapium [INN-French]; Ioduro de oxapio [INN-Spanish]; ANC 113; Espalexan (TN); Oxapium iodide (JP17/INN); SCHEMBL1316687; CHEMBL2107009; DTXSID6046925; s109; Tox21_112814; 2-Cyclohexyl-2-phenyl-4-piperidinomethyl-dioxolane-1,3 methiodide; AC-669; AKOS015969034; Tox21_112814_1; VA11456; N-Methyl-N-(2-cyclohexyl-2-phenyl-1,3 dioxolan-4-yl-methyl)-piperidinium iodide; NCGC00181356-02; DB-080725; FT-0656107; D01815; Q10859610; 1-((2-Cyclohexyl-2-phenyl-1,3-dioxolan-4-yl)methyl)-1-methylpiperidin-1-ium iodide; 1-((2-Cyclohexyl-2-phenyl-1,3-dioxolan-4-yl)methyl)-1-methylpiperidinium iodide; Piperidinium, 1-((2-cyclohexyl-2-phenyl-1,3-dioxolan-4-yl)methyl)-1-methyl-, iodide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
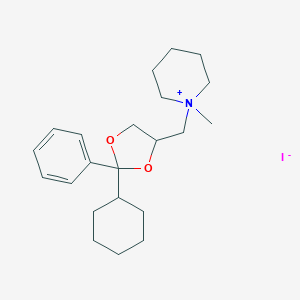 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 471.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
